Guide to 3M Masks / Respirators
Difference between mask and respirator- Selection of respiratory protection for Pollution hazards is typically based upon the airborne concentration of the substance that the wearer is exposed to, fit of the respirator and the hazard exposure limit of that substance. Selection of respiratory protection for Pollution hazards is typically based upon the airborne concentration of the substance that the wearer is exposed to, fit of the respirator and the hazard exposure limit of that substance.
Particulate Respirators | Surgical Masks | Comfort Masks |
|
|
|
|
|
|
Who will benefit from wearing a respirator?
• People with respiratory complications such as asthma or lung disease
• People who are obese or have diabetes
• People who have been diagnosed with a cardiovascular disease
• Children, teenagers and the elderly
• Expectant women and new mothers
Surgical N95 vs. Standard N95 – Which to Consider? |
|
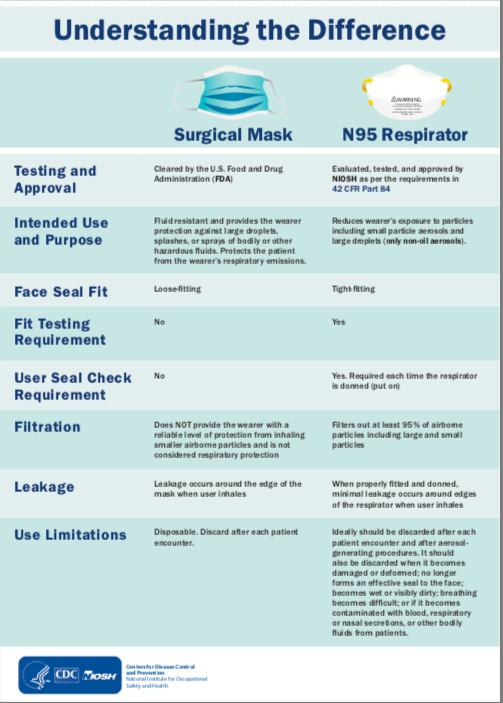
Many tasks performed by healthcare workers – such as patient intake and non-emergency patient evaluation – pose little risk of generating high-pressure streams of liquid and are not considered surgical procedures. For workers performing such tasks, a primary potential hazard to consider is airborne droplet containing viruses and bacteria, such as those generated by coughs and sneezes, which can be effectively filtered by a properly selected and worn N95 respirator. Therefore, if a healthcare facility is prioritizing respirator use – due to limited supply during a health emergency – they may want to consider prioritizing use of surgical N95 respirators for those healthcare workers requiring respiratory protection while performing surgery or other tasks that may expose them to high pressure streams of bodily fluid. The US Centers for Disease Control and Prevention (CDC), in their webpage Frequently Asked Questions about Personal Protective Equipment, says, “In times of shortage, only healthcare professionals who are working in a sterile field or who may be exposed to high velocity splashes, sprays, or splatters of blood or body fluids should wear these [surgical N95] respirators, such as in operative or procedural settings.”2 For other workers who will not be performing such surgical procedures and do not require protection from high-pressure streams of liquid, a standard non-surgical N95 (or similar) respirator can be worn to help reduce those workers’ exposure to patient-generated airborne viruses and bacteria.
The following chart demonstrates some key similarities and differences between three respirator models. The 8210 is a standard N95 respirator, while the 1860 and 1870+ are both surgical N95 respirators. 2 |
3M Personal Safety Division | |||||||||||||||||||||||||||||||
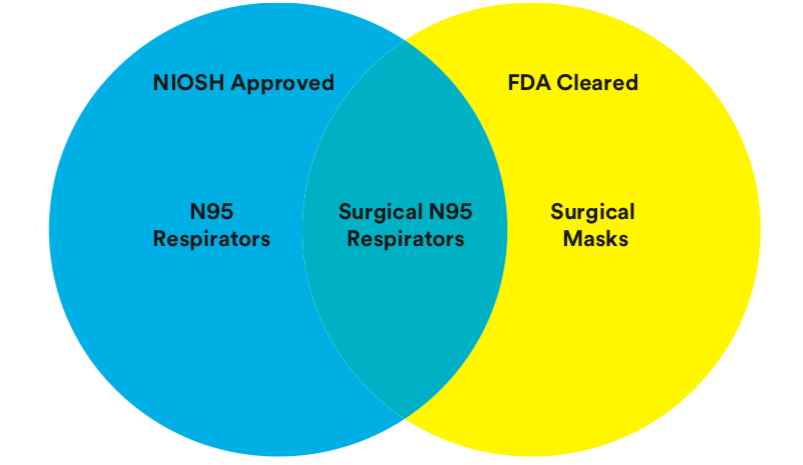
Notes – 1) ASTM F1862 is a standard test method for resistance of medical facemasks to penetration by synthetic blood. This test is required because during certain medical procedures, a blood vessel may occasionally be punctured, resulting in a high-velocity stream of blood impacting a protective medical facemask. The test procedure specifies that a mask or respirator is conditioned in a high-humidity environment to simulate human use and is placed on a test holder. Synthetic blood (2cc) is shot horizontally at the mask at a distance of 30 cm (12 inches). Surgical masks and respirators are tested on a pass/fail basis at three velocities corresponding to the range of human blood pressure (80, 120, and 160 mmHg). The inside of the mask is then inspected to see if any synthetic blood has penetrated to the inside of the facemask. Fluid resistance according to this test method is when the device passes at any level. N95 Particulate Respirators, 1860/1860S and 1870 What is a type N95 respirator? A. An air-purifying, particulate respirator is a personal protective device designed to help reduce the wearer’s inhalation exposure to certain airborne particles. N95 is one of nine filter classifications in the National Institute for Occupational Safety and Health (NIOSH) approval system. The Occupational Safety and Health Administration (OSHA) requires respirators be certified by NIOSH. In the NIOSH classification system, particulate respirators are given an N, R, or P rating. Each particulate respirator is also given a filter efficiency rating of 95, 99, or 100 when tested against particles that are the most difficult size to filter – approximately 0.3 microns in size mass median aerodynamic diameter (MMAD). NIOSH class 95 particulate respirator filters are certified to be at least 95% efficient; class 100 particulate respirator filters are certified to be at least 99.97% efficient. The most commonly used respirator in healthcare settings is an N95 filtering facepiece respirator. This class of respirator has an assigned protection factor (APF) of 10, which in essence means it will reduce contaminant exposures by a factor of 10. The APF defines the workplace level of respiratory protection that a respirator or class of respirators is expected to provide to employees when the employer implements a continuing, effective respiratory protection program as specified in the OSHA Respiratory Protection Standard, 29 CFR 1910.134. Q. Who is NIOSH? A. The National Institute for Occupational Safety and Health (NIOSH) is part of the Department of Health and Human Services and has responsibility for testing and certifying respirators. They are NOT responsible for regulating their use. Q. Who is OSHA? A. The Occupational Safety and Health Administration (OSHA) is part of the Department of Labor. OSHA establishes standards to ensure safe and healthful workplaces in the US. The OSHA standard that affects respirator use in healthcare facilities is the General Industry Respiratory Protection Standard 29 CFR 1910.134. OSHA is an enforcement agency and as such has the authority to cite and issue penalties to employers if applicable standards are not being followed. Q. Who is CDC? A. The Centers for Disease Control and Prevention, a federal Q. How is an N95 respirator different from a medical, surgical, or patient care mask? Medical, surgical, or patient care face masks, on the other of airborne hazards; therefore they should not be considered an equivalent substitute to government-approved respirators. Look for the N95 certification label on each respirator to assure NIOSH approval. Q. Can the 3MTM N95 Respirators be used in surgery?A. Yes. The 3M Particulate Respirators and Surgical Masks 1860/1860S and 1870 are cleared for use as a surgical mask by the FDA. They provide greater than 99% bacterial filtration efficiency against wearer generated particles according to the Modified Greene and Velsey test method. They are also fluid resistant to help reduce exposure to blood and body fluids. When worn properly and in combination with protective eyewear, they can help the facility comply with the OSHA Bloodborne Pathogen Standard. Q. What is BFE, and what does it measure? A. BFE stands for Bacterial Filtration Efficiency. This test evaluates how well a surgical mask can prevent biological particles from being expelled by the wearer into the environment. Bioaerosol particles generated during the BFE test are “large,” on the order of 1 to 5 microns in size. For comparison, particles used for respirator filter efficiency tests are much smaller, approximately 0.3 microns MMAD in size. The BFE test is a relative indicator of the performance of a medical, surgical Q. Are the 3MTM N95 respirators appropriate for reducing exposure to M. tuberculosis? Q. Can respirators help protect you from biological agents such as bacteria or viruses? Q. What is the risk of inhaling biological particles that have been collected by the respirator filter? Q. Can the 3MTM N95 Respirators be used during the administration of Ribavirin (VirazoleTM)? Q. Can 3MTM N95 Respirators be used during the administration of pentamidine? Q. Do the 3MTM N95 Respirators contain fiberglass material? Q. Can 3MTM N95 Respirators be used when caring for patients known or suspected to have measles (rubeola) or chickenpox (varicella)? Q. Should a respirator be worn by a patient with a compromised respiratory system? Q. Can the 3MTM N95 Respirators be used for laser and electrocautery procedures? Q. What is the difference between the 1860/1860S and the 1870? small) and the 1870 model. The 1860 is a traditional cup shaped respirator where as the 1870 is a three-fold, flat panel respirator. The 1870 provides the benefits of a cup-style product with the convenience of the flat-fold products. Both the 1860/1860S and the 1870 have two elastic headbands (latex free) and a malleable nose clip and foam strip to help provide a secure, customized fit for a broad range of face shapes and sizes. Q. Are the fitting instructions for the 1870 respirator different than for the 1860/1860S? Q. Do the 3MTM N95 Respirators contain latex? Q. Do the 3MTM N95 respirators require fit testing? Q. How often must fit testing be done? A. OSHA, under its respiratory protection standard 29 CFR 1910.134, requires that fit testing be conducted when the respirator is first issued to an employee and at least annually there after. Additional fit tests must be conducted whenever a different respirator (size, style, model, or make) is used and if changes in facial structure of the wearer develop that could affect respirator fit. Q. Are multiple sizes of respirators needed? A. Multiple sizes of respirators are not mandatory. Multiple sizes or alternative facepiece designs can provide the individual with additional options for obtaining a good fit and seal. What is important is that the respirator fit the wearer. 3M offers the 1860 model in both a standard size and a small size (1860S). The 1870 model is only available in one size and is designed to fit a wide range of face sizes. Q. Is a user seal check or fit check the same as a fit test? A. No. A user seal check is a quick (6–10 second) procedure, performed by the wearer, to determine that the respirator is properly molded to the face prior to entering a contaminated area. A user seal check is required each time a respirator is donned or adjusted. It does not take the place of a fit test. A fit test is used to evaluate the fit of a particular model and size of tight-fitting respirator on an individual before use. The respirator program supervisor or trainer conducts the fit tests. Q. How is a user seal check/fit check performed? A. To perform a user seal check on the 1860/1860S or the 1870, don the respirator according to the user instructions. Next, place both hands completely over the respirator and exhale. If air leaks between the face and the face seal of the respirator reposition it and readjust the nose clip for a more secure seal. If air leaks around the respirator edges, adjust the position on the face and the straps along the sides of the head and recheck fit. If a proper fit cannot be achieved, do not enter the area requiring respiratory protection. See specific product user instructions for the most current user seal check/fit check instructions. Q. Can the 3MTM N95 Respirators be worn with facial hair (i.e. beard or stubble)? Q. Can disposable respirators be shared between people? A. No. Disposable respirators should never be shared and when not in use should be stored according to the facility’s infection control policy and procedure and OSHA regulations. Q. How long can the 3MTM N95 respirators be used?A. The 1860/1860S and 1870 may be used until damaged, breathing becomes difficult, or contaminated with blood/body fluids. If contact transmission is of concern, it may be appropriate to dispose of immediately after each use. Otherwise, it may be stored and reused according to the facility’s infection control policy and procedure. Q. How should the 3MTM N95 Respirators be stored?A. According to OSHA all respirators must be stored to protect them from damage, contamination, dust, sunlight, extreme temperatures, excessive moisture, and damaging chemicals. Respirators must also be stored to prevent deformation of the facepieces. Q. Can I store and/or carry the 1870 in my pocket? A. 3M does not recommended storage of the 1870 respirator in pockets once used, since the respirator may be damaged or become contaminated if out of the package. |