SARS-CoV-2 is difficult to diagnose early in infection as patients may be asymptomatic or have mild, non-specific symptoms. The current standard for diagnostic molecular is quantitative reverse transcription PCR (qRT-PCR), which requires expensive equipment and trained personnel only available in limited laboratories.
Reverse transcription loop-mediated isothermal amplification (RT-LAMP) is a one-step technology with fewer barriers to use than qRT-PCR; reagents are inexpensive and can be stored at room temperature, the technique works with a range of sample types, and it has been found to be highly specific, sensitive, and fast for other infectious diseases.
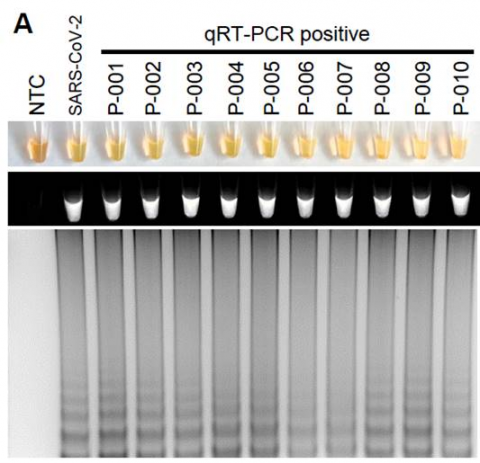
In the new study, researchers developed an RT-LAMP diagnostic test for SARS-CoV-2 and measured the effectiveness of the test on blood, urine, saliva and mouth swab samples that had been spiked with different amounts of SARS-CoV-2 genetic material. The test was also used on clinical samples from COVID-19 patients.
The RT-LAMP test for SARS-CoV-2 successfully detected the virus in all human sample types that were spiked with SARS-CoV-2 genetic material, within 30 to 45 minutes, with an estimated limit of detection of as few as 304 viral copies in a sample. Importantly, the test did not detect virus in samples spiked with RNA other coronaviruses, demonstrating specificity for SARS-CoV-2. In clinical mouth swab samples, RT-LAMP was positive for 95% (19/20) of samples positive by qRT-PCR and negative for 90% (18/20) of samples that were negative by qRT-PCR. The researchers note that those discrepancies between the methods could represent either false positives by the RT-LAMP, contamination or increased sensitivity of RT-LAMP compared to qRT-PCR. The current study was not powered to determine sensitivity in a clinical population.
This method can rapidly detect the virus for COVID-19 in clinical samples without expensive equipment.”
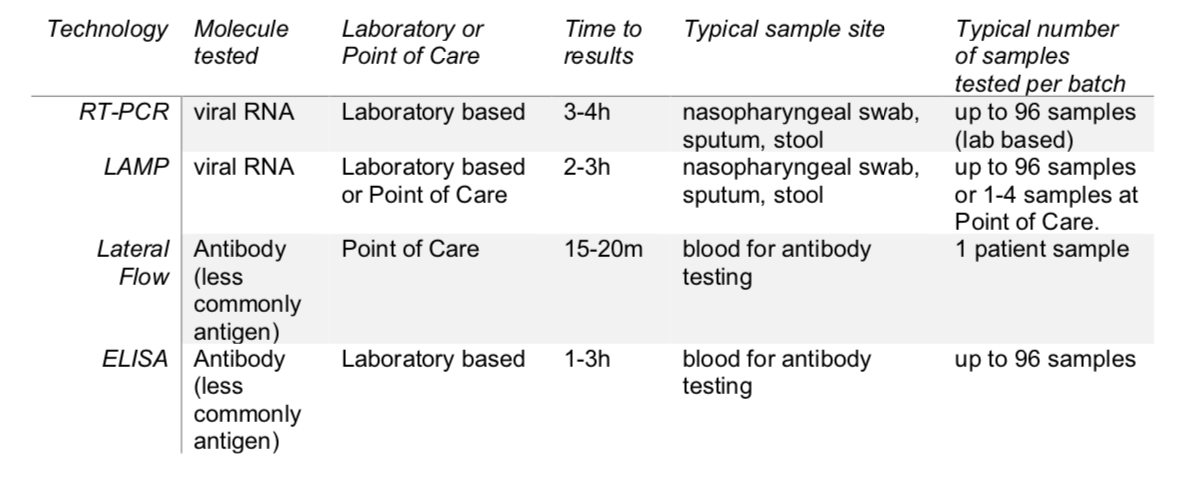
What tests could potentially be used for the screening, diagnosis and monitoring of COVID-19 and what are their advantages and disadvantages?
VERDICT
Many diagnostic tests for coronavirus disease 2019 (COVID-19) are available so far, with more gaining emergency approval every day. These tests are largely based on four different techniques, 1) reverse transcription polymerase chain reaction (RT-PCR) — the current standard test for COVID-19, 2) loop-mediated isothermal amplification (LAMP) — a simple, but less developed testing method, 3) lateral flow — hand-held single-use assays providing results for an individual patient in as short as 15 minutes, and 4) enzyme-linked immunosorbent assay (ELISA) — quick and technically simple assays that are easily read and offer relatively high throughput.
BACKGROUND
A range of molecular techniques ranging from central laboratory testing to point-of-care tests are under development or already available for the diagnosis and management of COVID-19 patients. These techniques, while well-known to researchers and clinicians with a background in deoxyribonucleic acid (DNA) amplification, as well as antibody and antigen assays, may be relatively unknown to the broader community. Here we outline and explain the basic principles of the main COVID-19 diagnostic tests currently in use or in trials.
GLOSSARY
Key terms used in this document (highlighted in bold) are described here:
Antibody — Antibodies are protective proteins produced naturally in response to seeing foreign structures (antigens), e.g. during a viral infection. The body produces early `prototype’ antibodies (IgM) with intermediate strength for binding to the virus. These are able to start working to clear virus about five days after a new infection started. Typically eight to ten days after the start of the infection, IgG antibodies with high binding strength, can work to help more rapid virus clearance. Antibodies act by developing a matching contoured surface to stick to antigens, using a sophisticated selection process to amplify antibodies with the best surface match and strongest binding.
Antigen — An antigen is a structure recognised as foreign by the human body that triggers the immune system to produce self defence systems to clear pathogens from blood and tissues. Antigen clearance is spearheaded by antibodies and white blood cells.
COVID-19 — Coronavirus disease-19 — A respiratory illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), colloquially referred to as coronavirus. The family of coronaviruses includes not only SARS (-1 and -2) and MERS, but also milder forms (causing 15–20% of common colds).
DNA — Deoxyribonucleic acid — The molecule that humans and most organisms use to store their genetic code. DNA contains the information that cells use to make proteins. It is formed from two strands that bind together forming a helix shape.
Enzyme — An enzyme is a protein that speeds up chemical reactions by adapting or changing other proteins and molecules.
Fluorescence — This is the light given off by certain molecules when they absorb energy.
Herd-Immunity — This method of protection from the spread of disease provides population immunity by vaccinating a large percentage of the population, thereby protecting those who are not immune by reducing their chance of exposure to the disease.
RNA — Ribonucleic acid — In humans RNA has many functions, but a main one is to copy sentences of DNA to make proteins. Some viruses use RNA instead of DNA to store their genetic code, including the virus that causes COVID-19.
Sensitivity — The ability of a diagnostic test to give a positive result when it is supposed to be positive.
Serum — Serum is a yellowish fluid which is part of the blood. It is the liquid component of blood once the blood cells have been removed.
Specificity — The ability of a diagnostic test to indicate a negative result when it is supposed to be negative.
Swab — A small piece of gauze or other absorbent material attached to a stick used for collecting patient samples. Both long and short swabs are used in coronavirus testing. Long swabs are used by a clinician to take nasal swabs, but short swabs can be used by patients themselves. Different protocols may require different swab types.
Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
Technology overview – PCR is a very common scientific technique that has been widely used in research and medicine for around 20–30 years to detect genetic information. RT-PCR is a special version used when RNA is being detected and it is now being used as a test to detect SARS-CoV-2, the virus causing COVID-19. This type of test has frequently been used as a frontline test for COVID-19 as it directly tests for the presence of the virus RNA. RT-PCR tests are fairly quick, sensitive and reliable, capable of producing results in 3-4 hours, although this usually takes longer if samples must first be sent to specialized external laboratories (6–8 hours on average). Many diagnostic and research companies produce RT-PCR products, tests and machines so the technology is widely available. Some RT-PCR tests are developed as an `all in one’ kit, reducing laboratory handling and potential for contamination.
How it works – Once a sample has been collected, chemicals are used to remove any proteins, fats and other molecules, leaving only RNA behind. This will be a mixture of a person’s genetic material as well as any viral RNA that might be present. The test kit enzymes copy the RNA to DNA, which is amplified to allow virus detection by using a PCR machine which cycles the test temperature so that roughly 35 billion copies of viral DNA are made for each viral RNA strand that was originally present. Fluorescent markers are typically used to bind to the amplified DNA and produce light, which can be read by the machine to produce the test result. If the intensity of the light produced within the sample reaches a certain threshold, this is classed as a positive test. The number of PCR temperature cycles that were required before the fluorescence threshold was reached is recorded and gives an estimate of how much virus was present in the patient sample.
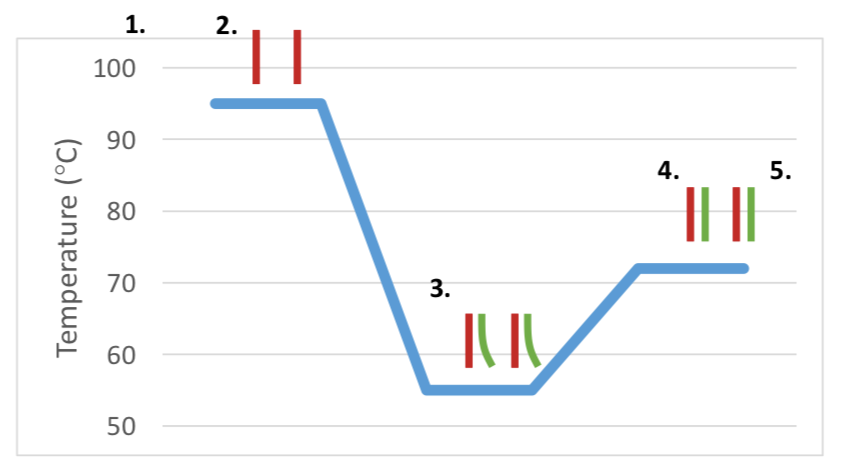
- Viral RNA is copied to make a DNA copy of the virus using small `primer’ DNA strands that are designed to copy only SARS-CoV-2 virus RNA.
- The viral DNA copy is heated to 95°C, to open up its DNA strands.
- The reaction is cooled to 55°C which causes the separated strands to bind with small complementary `primers’ of DNA as well as fluorescent markers.
- Heating the reaction up to 73°C allows the new stands of DNA to be made and extend producing 2x the amount of DNA compared to that in step.
- The heating and cooling cycles (steps 2–4) are repeated around 30 times, each time doubling the amount of viral DNA that forms.
- This can then be detected by the machine Temperature (°C)

RT-PCR detects whether or not viral RNA is present in samples from a patient. It does this by capturing and amplifying regions of the virus’ genetic material, usually the Spike protein, N protein or Envelope (see picture). Commonly these samples are taken from the nose or throat using either long or short swabs, but samples can be collected in other ways too. Collecting samples from where the virus is shedding or multiplying, improves the accuracy of the test. An RT-PCR test is highly sensitive and fairly reliable if performed on a sample from an infected part of the body whilst an active infection is occurring.
- Positive Test Result: — A positive PCR result means that the person the sample was taken from is currently infected by the virus.
- Negative Test Result: — A negative PCR result could mean that the person is not currently infected by this virus, the virus is not present at the site the sample was taken from, the sample taken was of poor quality, or that it is too early, or too late in the infection to detect replicating virus. This is why negative test results require new patient samples to be taken a few days later to reduce the chance of incorrectly missing an infected person.
The RT-PCR test cannot detect if a person has had the virus and then cleared it after the end of the COVID-19 disease, i.e. whether a person had the disease, as it only detects when active virus is present.
Advantages and limitations
Advantages:
- RT-PCR is accepted by scientists and medical staff as a robust and well documented technique.
- With RT-PCR being so common in research and medicine, the technology is already in place to test for COVID-19.
- RT-PCR can detect current infections of disease, allowing medical staff to determine who is currently infected and who is not.
To measure the viral RNA, it is converted to DNA, copied many times using repeated temperature cycles in a PCR machine and then fluorescent markers are used to detect the virus. If the amount of fluorescence goes above a certain level, this confirms that the virus is present. The number of temperature cycles the machine performs to reach this threshold is recorded to estimate how much virus was present in the patient sample. The lower the number of cycles, the more virus was present.
What does the result mean?
Disadvantages:
- RT-PCR relies on capturing and detecting the virus and so it is possible to miss patients who have cleared virus and recovered from disease.
- The distribution of virus across the respiratory tract varies between patients, so even if a person is infected, the virus may only be detectable in sputum or nasopharyngeal swab but not necessarily at both locations at the same time.
- RT-PCR for COVID-19 can only tell if a person is currently infected with this particular coronavirus. It can’t provide information on other diseases or symptoms.
Loop-Mediated Isothermal Amplification (LAMP)
Technology overview- Loop-mediated Isothermal Amplification (LAMP) is a similar process to RT-PCR, but instead of using a series of temperature changes to produce copies of the viral DNA, LAMP is conducted at a constant temperature of 60–65°C. The amount of DNA produced in LAMP is much higher than in RT-PCR and a positive test result can be seen visually without requiring a machine to read the results. LAMP is a newer technique compared to RT-PCR, but is technically simple and easy for a trained scientist to perform, making it a potentially useful technique for detection of COVID- 19. As it is a newer technology, there is less evidence on its use, but diagnostic companies are currently performing clinical trials to support it.
How it works – LAMP assays for COVID-19 start with the collection of samples from the nose or throat using a swab, but can also use samples collected using other methods too such as mucus produced from hard coughing. Like RT-PCR, the viral RNA in the sample is converted to DNA which allows it to be copied. The amplification of the viral DNA using LAMP technology and reagents can be detected when the reaction mixture is turned cloudy due to the production of a chemical called ‘magnesium pyrophosphate’. As this cloudiness can be seen by the naked eye, it allows foreasy diagnosis of COVID-19 by scientists and clinicians. The accuracy of the results can be improved by using special fluorescent dyes or colour changing dyes in the reaction mixture. As the dyes interact with the viral DNA, the intensity of the light or colour change can be measured to give the approximate number of viral RNA molecules that were initially in the sample.
What does the test detect? – Like RT-PCR, LAMP detects whether or not viral RNA is present in samples from a patient.It does this by capturing and amplifying regions of the virus’ genetic material, usually the Spike protein, N protein, Envelope or multiple regions at once (see virus diagram in RT-PCR section above). Samples can be collected in the same way as they are for RT-PCR, usually from the nose or throat using long or short swabs, or through mucus produced when a person coughs strongly. In order to measure the viral RNA, it must be converted to DNA using an enzyme and copied many times. The results of the test are determined based on the cloudiness or colour change of the reaction mixture.
What does the result mean?- Like RT-PCR, LAMP tests are highly sensitive and reliable if performed on a sample from an infected part of the body whilst an active infection is occurring.
- Positive test result: — A positive LAMP result means that the person the sample was taken from is currently infected by the virus.
- Negative test result: — A negative LAMP result could mean that the person is not currently infected by this virus, the virus is not present at the site the sample was taken from, or that it is too early, or too late in the infection to detect replicating virus. This is why when test results are negative, new patient samples are taken a few days later to reduce the chance of incorrectly missing an infected person.
LAMP tests cannot detect if a person has had the virus and then cleared it after the end of the COVID-19 disease, i.e. whether a person had the disease, as it only detects when active virus is present.
Advantages and limitations
Advantages:
- LAMP is a quick technique that can produce results in 2–3 hours.
- Results can be read by eye.
- The simple and cheap method can be done within hospital laboratories, improving the time between taking a sample and getting a diagnosis.
- LAMP can detect current infections of disease, allowing medical staff to determine who is currently infected and who is not.
Disadvantages:
- The technology is newer than RT-PCR and does not have such a large background of research behind it. Tests using LAMP technology for COVID-19 are still being assessed in clinical settings.
- The science behind building these tests is more difficult than RT-PCR.
- LAMP tests rely on capturing and detecting the virus and so it is possible to miss patients who have cleared virus and recovered from disease.
- The distribution of virus varies across the respiratory tract between patients, so even if a person is infected the virus may only be detectable in sputum or nasopharyngeal swab but not necessarily at both locations at the same time.
- LAMP tests for COVID-19 can only tell if a person is currently infected with this particular coronavirus. It can’t provide information on other diseases or symptomsand does not tell staff if a patient has been previously infected with the virus or if a patient has any immunity to the virus.
Lateral Flow / Colloidal Gold Immuno Chromatography
- Technology overview-Lateral flow assays have commonly been referred to as ‘Antibody tests’ in the media as they are currently used to detect antibodies to disease in a patient’s blood. The technology is also being tested for antigen use too.
Lateral flow assays use the same technology commonly used for pregnancy tests. Lateral flow tests can detect antibody to virus from patient blood indicating that the patient has COVID-19 or has recovered from COVID-19. Less commonly, lateral flow tests can be used to detect the presence of active virus by detecting virus proteins directly. Antibody lateral flow tests for SARS-CoV-2 are produced as test kits used by a specialist or clinician rather than by patients themselves. They require a drop of patient blood, either from a vein or from a small finger prick, similar to a finger prick test used for blood sugar monitoring in certain types of diabetes. These types of tests work very differently to RT-PCR and LAMP techniques and detect the patient’s immune antibody response to the virus rather than detecting the virus itself. Lateral flow antibody tests can be completed rapidly and the tests can be produced cheaply, so multiple diagnostics companies are working hard to develop lateral flow tests for SARS-CoV-2. A major advantage for this type of test is also the ability to see if patients are currently infected or have recovered from COVID-19 even if they have fully recovered and cleared the virus months ago. However, it cannot distinguish between an active and a previous infection.
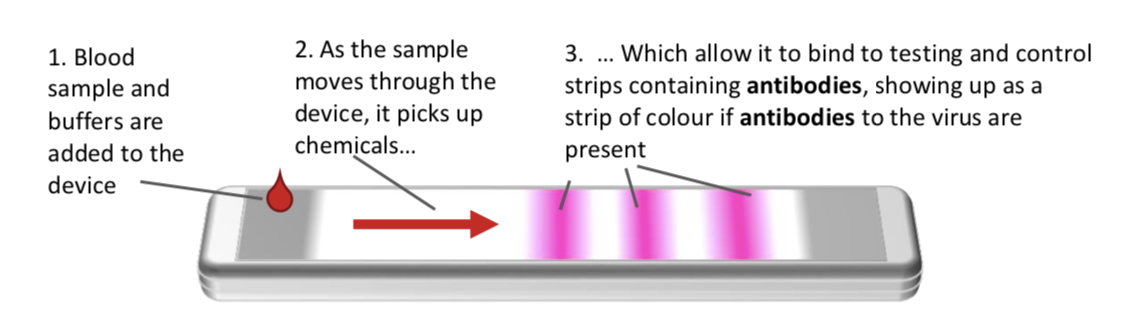
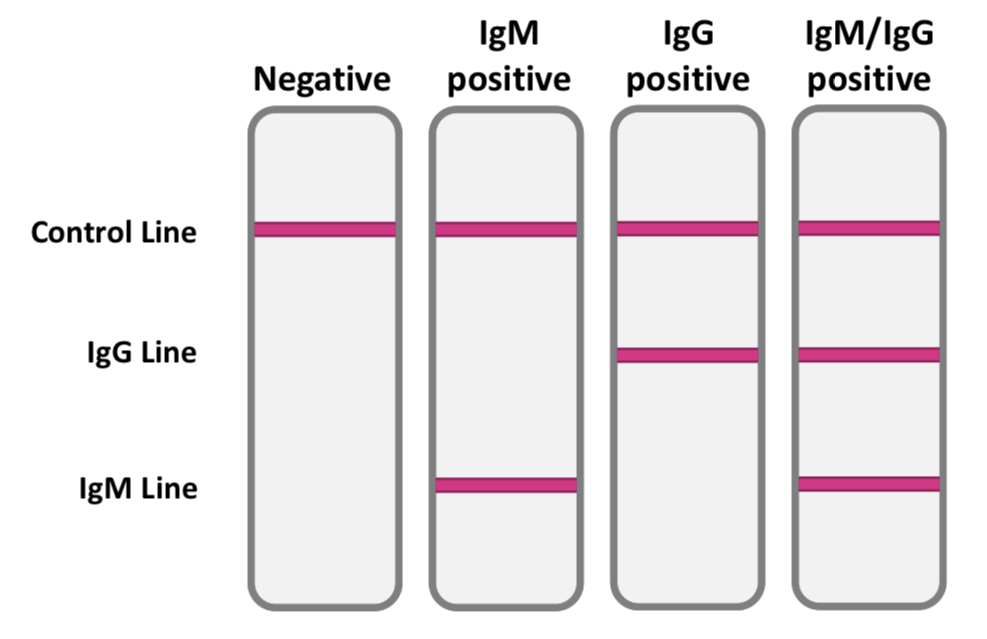
How it works-Lateral flow immunoassays for COVID-19 are simple devices that can detect antibodies in the blood. A small sample of patient blood is taken from a vein or from a finger-prick by a clinician and dropped onto a spongey pad within the test device. A few drops of a diluting liquid called a‘buffer’ are added to help the blood sample flow across the device. As the sample moves through the device, antibodies against SARS-CoV-2 that are present in the sample will attach to chemicals in the device, capturing the antibodies on the test and control lines. This capturing and binding process results in a colour change along the test and control lines which can be seen by eye, producing one, two or three lines depending on the type of antibodies are present (IgM or IgG).
- Blood sample and buffers are added to the device
- As the sample moves through the device, it picks up chemicals…
- Which allow it to bind to testing and control strips containing antibodies, showing up as a strip of colour if antibodies to the virus are present
What does the test detect-Lateral flow immunoassays for SARS-CoV-2 detect two types of protective antibodies that are produced by the body when the immune system recognises a foreign structure, in this case SARS-CoV-2, the virus causing COVID-19. These antibodies help fight the disease and remain in the blood for months after the virus and disease is cleared. The presence of antibodies in the body is often referred to as immunity or that a person is immune to a virus, as these antibodies protect against re- infection and return of the same disease. When we are infected by virus, our immune system produces early `prototype’ antibodies(IgM) with intermediate strength binding to virus, that are able to start working to clear virus about 5 days after a new infection. Typically at 8 to 10 days after infection, IgG antibodies with high binding strength, can work to help more rapid virus clearance. Antibodies act by developing a matching contoured surface to stick to foreign antigens, using a sophisticated selection process to amplify antibodies with the best surface match and strongest binding. Antibody lateral flow immunoassays can be designed to detect IgM or IgG alone or both together. Antigen lateral flow immunoassays are an even newer technology with additional scientific and technical challenges which mean they are not likely to be developed during the pandemic period. These antigen assays detect the virus directly without the amplification steps of RT-PCR and LAMP, and like those tests are only able to detect current active viral infection but not past infection.
What does the result mean-Antibody lateral flow immunoassays detect antibodies to the virus in the blood. They don’t detect the virus itself. The ability to detect the immune system response reliably using only one sample (blood) is a huge advantage, as is the amplification of the detection signal generated by the body immune response. Using the antibody response alone does not allow distinction between individuals who are currently infected and those who have cleared the virus infection. Antibody tests provide a hugely important ability to detect past infection with virus to identify people who were asymptomatic, people who have cleared the virus and so no longer risk being infected or spreading the virus to others. In addition, antibody tests are critical for assessing population spread of the virus and the level of ‘herd’ immunity in the population. This is important for understanding the potential consequences of lifting or enforcing measures to control the virus such as quarantine, social distancing, school and workplace closures. The antibody IgG and IgM lateral flow immunoassay tests are very simple to read:
A control line must appear to show that the assay has worked correctly. Then, test lines will appear if either of the antibody types are found in the sample. The appearance of lines for IgG or IgM, or both indicate a positive test — showing that the patient has been infected with the COVID-19 coronavirus.
Advantages and limitations
Advantages:
- Lateral flow assays are extremely quick per patient, giving results in just 15 minutes.
- Testing levels of antibody in blood allows a single patient sample from one accessible part of the body where sampling is non-invasive to be tested for presence of virus.
- These tests require very little training to perform and don’t rely on specialist laboratories or scientists to analyze.
Disadvantages:
- The technology is new and the evidence for its accuracy in coronavirus diagnosis is still being evaluated.
- So far, available lateral flow tests can only determine if a patient has at some point been infected with COVID-19. Further testing would be needed to check if a patient is currently infected. Future versions of this technology might allow clinicians to detect current infections.
- Lateral flow tests are more expensive and time consuming for large batch testing than specialist laboratory based antibody tests such as ELISA.
Enzyme-Linked Immunosorbent Assay (ELISA)
Technology overview – An Enzyme-Linked Immunosorbent Assay (ELISA) is a common biochemical technique that can be used to detect antigens or antibodies, depending on the type of test used. ELISAs use enzymes linked to antibodies that can attach to the molecule that is being tested for and cause a colour change that can be measured by a specialized machine. The strength of the colour change gives scientists and clinicians an idea of the number of molecules of interest in the sample. ELISAs can be done as standard batches of up to 96 assays completed at the same time, allowing cheap and time effective method for batch testing of large numbers of patient samples at the same time. This technology could help speed up the number of patients that can be tested for SARS-CoV-2. The most effective ELISA assays in monitoring prior infection detect antibodies against SARS-CoV-2. Future ELISA could be used to test for active virus infection by detection of virus protein (antigen) testing, but this testing will not be as accurate and is as yet unproven.
How it works – An ELISA detects antibodies produced in patient blood due to infection with SARS-CoV-2. The whole test can be performed in one tube or well and involves mixing patient samples, antibodies, antigens and enzymes together with a colour changing molecule. The example below describes a typical antibody ELISA test.
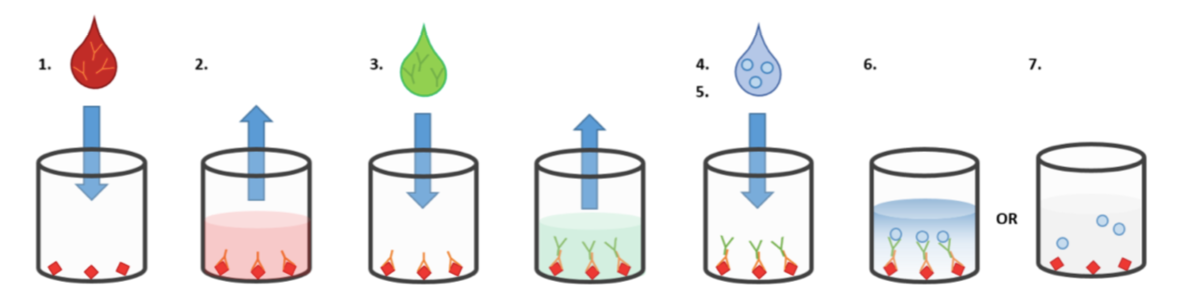
- A patient sample of blood or serum (which may contain antibodies to SARS-CoV-2) is added to a well containing SARS-CoV-2 specific antigen.
- The patient antibodies to SARS-CoV-2 stick to the SARS-CoV-2 proteins coated on the bottom of the well and the rest of the liquid sample is washed off.
- Other laboratory-produced antibodies with enzymes attached are added and stick to patient antibodies present in the well as a `second layer’. Excess antibody with enzyme attached is washed off, so enzyme is only present in wells when a patient has produced antibodies to COVID-19.
- A special colourless molecule is added to the well.
- In wells containing samples from patients who have been infected with COVID-19 and so have antibodies to the virus, the `second layer’ antibodies with enzyme act on the special colourless molecule causing it to rapidly change colour indicating a positive test.
- This colour change can be read either by eye or by a machine called a spectrophotometer.
- If the patient had not been infected with COVID-19, the enzyme-linked antibody would not stick to anything in the well and the colourless molecule would not change colour. This would be a negative test.
What does the test detect-ELISA tests to detect antibodies are detecting the antibody response to COVID-19 infection. Detecting antibodies to SARS-CoV-2 could tell a clinician if a patient has been infected with COVID-19, either currently or in the past. However infected patients will not be detected immediately on infection, but only when the immune system to the virus can be detected in blood, roughly 5 days after infection for a test detecting IgM antibodies, which is about the same time that symptoms occur. Current knowledge suggests once a person has been infected with virus, their immune system will prevent a future infection with the same virus. This antibody ELISA test provides very important information for diagnosis, management and recovery from COVID-19 and will also help researchers evaluate how many people in the population have been infected, which is important to planning infection control.
What does the result mean- An antibody test using ELISA would show a positive result (colour change) if the patient has antibodies to COVID-19. This might not mean that they currently have the virus, only that they have had it at some point. This is because antibodies stay in the blood even after the infection is gone to help provide the body with immunity if they come into contact with the virus again. A negative result (no colour change) would mean that the patient has not been infected with COVID-19 and may have no immunity against it. ELISA antigen tests may be developed in the future to detect current infections. Such an antigen test using ELISA would show a positive result (colour change) if a patient has COVID-19 in their blood. This would indicate that the patient is currently infected with the COVID-19. A negative result (no colour change) would indicate that no COVID-19 antigens were found in the patient’s sample. This could mean that the patient does not have COVID- 19, but might also mean that they are too early in their infection to be positive. If they have symptoms of COVID-19 they should be tested again a few days later to make sure.
Advantages and limitations
Advantages:
- ELISA is a simple and cheap laboratory technique.
- ELISA is well established and documented within science and medicine.
- Results can typically be produced within 1 to 3 hours of collecting a patient sample.
- Because it is so quick to perform it can be done in a hospital laboratory, cutting down the time to diagnosis.
- ELISA testing can be completed on multiple samples at once, so it can be used for rapid testing scaled up to test larger numbers of patients.
Disadvantages:
• ELISA tests are not yet well established for SARS-CoV-2 COVID-19 testing, although many companies are working hard to produce them and test them in patients.
CONCLUSIONS
- Four main types of tests are being used or being developed to test for SARS-CoV-2, the virus causing COVID-19.
- These tests are have been developed, validated and produced.
- Each test type has its own distinct advantages and disadvantages inherent to the underlying technology.
- A combination of testing types used at different times may be useful for patient management and population pandemic control of COVID-19.
- While individual tests differ in their properties, commonly the types of test listed in this document have the following profiles: