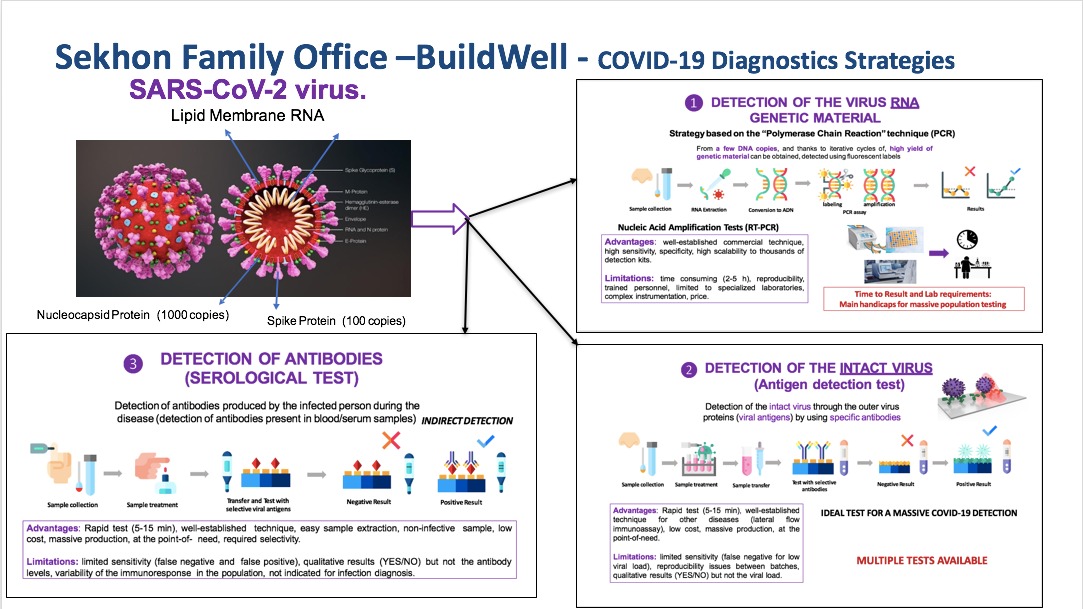
TESTING KITS AND SWABS
Please see below specifications and pictures
We are stockiest and suppliers of PCR / Antibodytesting kits from reputed brands with a manufacturing capacity of 0.5 Mn / week – The Detection Process. In the process of infection, antibodies appear in the human immune system. IgM is the first antibody in the human immune system, usually within one week, IgG antibody within 2-4 weeks. Detection of novel coronavirus specific IgM and IgG antibodies in acute infection stage has the advantages of high sensitivity, early diagnosis, and can be used to determine whether the suspect is infected or not. Therefore, detecting novel coronavirus novel coronavirus IgM and IgG antibodies is of great clinical significance in controlling the large-scale transmission of new coronavirus. IgM antibody is the earliest antibody in human immune system, which is a sign of recent infection. IgG antibody is later than IgM antibody, which is a sign of previous infection.
- SARS-CoV-2 / COVID-19 Fluorescent PCR.
- COVID-19 Antigen Detection rapid immunochromatographic assay test.
- SARS-CoV-2 / COVID-19 IgG Antibody Assay test kit(Colloidal gold immunochromatography).
- SARS-CoV-2 / COVID-19 IgM Antibody Assay test kit(Colloidal gold immunochromatography).
- SARS-CoV-2 / COVID-19 IgM/IgG Antibody Assay test kit(Colloidal gold immunochromatography).
- Test sample: serum, plasma, whole blood;
- Rapid screening of coronavirus infection within 15 minutes;
- No need for instruments and equipment, suitable for various on-site screening;
- Reduce the risk of sampling infection in hospital;
- High sensitivity and specificity;
- The reagent box shall be transported and stored at room temperature;
- It is of great significance for epidemiological investigation to clearly identify the past infected persons.
- Early diagnosis and treatment, shorten the course of disease.
- Reduce the workload of designated hospitals.
- Detection target 2019-nCoV ( COVID-19 )
- Target region Orf1a, N gene
- Detection technology Real-Time OneStep RT-qPCR
- Specimen type – Nasopharyngeal
- swab, Oropharyngeal swab, Sputum
- Compatible instruments* CFX96™ Real-Time PCR System (Bio-Rad)
- ABI 7500 / 7500 Fast Real-Time PCR System (Applied Biosystems)
- PCR running time – 120 mins
- Simple & Rapid detection system: OneStep Multiplex RT-qPCR based detection.
- HotStart PCR: high-specificity-.
- Reliable system: automatic PCR control (not Internal control).
- Easy-to-use master mix: just adding template and Primer/Probe Mix.
- Positive control included (Plasmid)
Coronavirus Antigen Rapid Test Kit
– Our coronavirus antigen test kit is a lateral flow immunoassay for the qualitative detection of SARS-COV-2 antigen (nucleocapsid protein) in upper respiratory samples with nasal swabs or saliva during the acute phase of infection. An uncut sheet format is available.
Features
- – 15-minute rapid detection
– Easy-to-operate coronavirus antigen test
– Less-invasive nasal (NS) swab sample collection
– CE-IVD marked
– Available in 1/2/5/20 tests/box
COVID-19 Antigen Rapid Test Principle
The coronavirus antigen rapid test kit is a lateral flow assay that qualitatively detects the presence of nucleocapsid (N) protein in upper respiratory samples (nasal swabs). This lateral flow assay is designed with the sandwich immunoassay format. When the specimen is added onto the sample pad of a test cassette, coronavirus N protein binds with colloidal gold-labeled SARS-CoV-2 N protein antibody to form an antibody-antigen (Ab-Ag) complex. The Ab-Ag complex is captured by SARS-CoV-2 N protein antibody (Rabbit monoclonal antibody) when migrating to the test line under capillary action. A red-colored band will appear on the test line, which indicates the specimen is COVID-19 nucleocapsid protein positive. No color band will appear on the test line if the specimen does not contain any coronavirus antigen (N protein), or the antigen level is below detection limit.
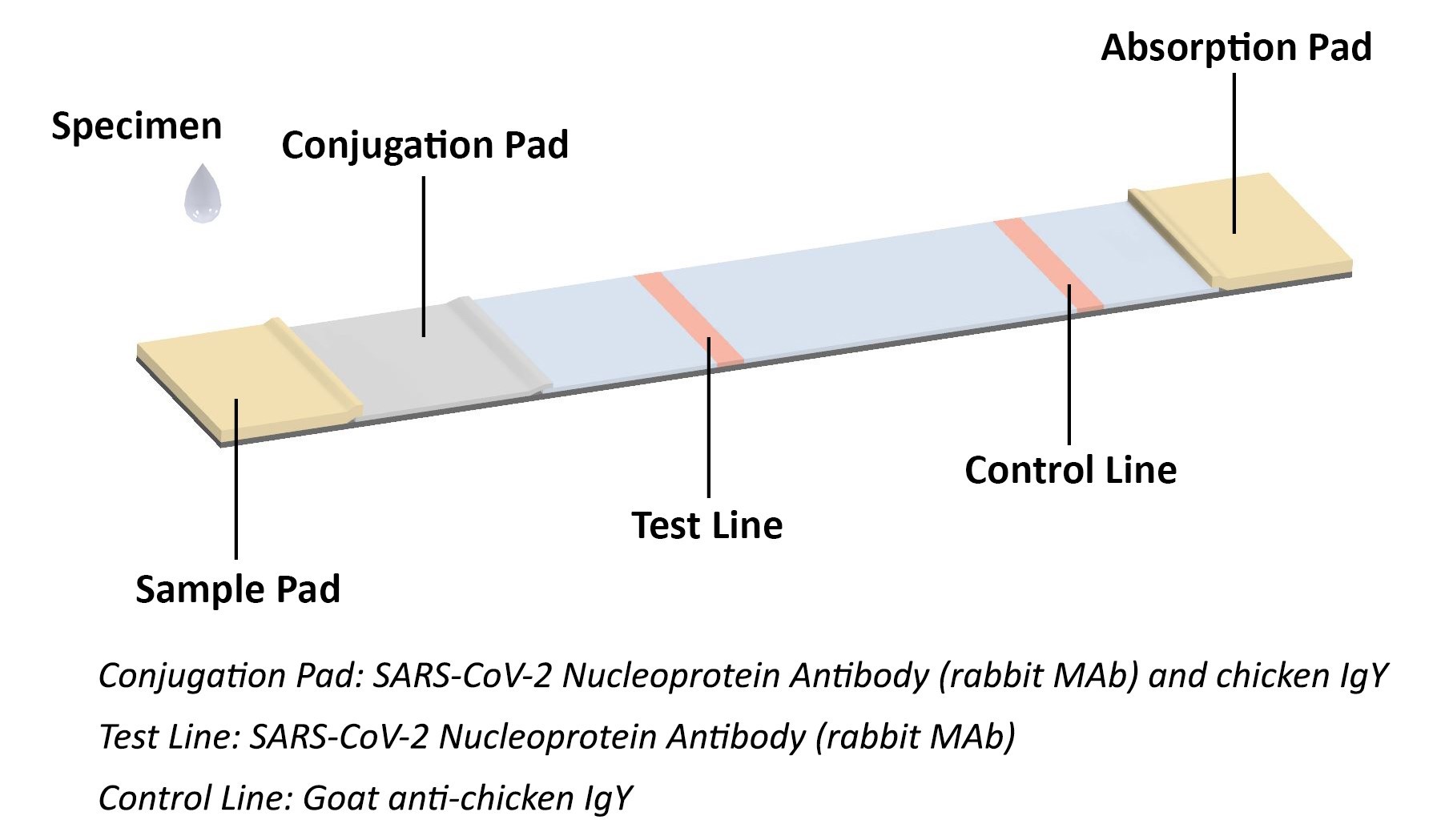 Coronavirus Antigen (Ag) Rapid Test Kit Principle
Coronavirus Antigen (Ag) Rapid Test Kit PrincipleThe conjugation pad also contains colloidal gold-labeled chicken IgY, which is captured by Goat anti-chicken IgY on the control line as procedural control. A colored band on the control line represents the proper liquid flow through the cassette; the absence of a colored band on the control line indicates insufficient sample or buffer volume.
- Test time – Within 15 mins
- Specimen-Whole blood/Serum/plasma.
- Storage temperature 2-30℃/36-86℉
- This kit is a rapid immunochromatography test designed for the qualitative presumptive detection of specific IgM and IgG to 2019 novel coronavirus (nCoV) in humoral fluid.
- Standard COVID-19 IgM/IgG Duo Test Kit is a rapid immunochromatography test designed for the qualitative presumptive detection of specific IgM and IgG to SARS-CoV-2 in humoral fluid.
- Rapid testing for SARS-CoV-2 antibodies within 10 minutesJust 10ul of specimen : Whole blood, serum , plasmaSuitable for Point of Care Testing.
- No need for extra equipment.
- MOQ – 50,000.
- Lead Times – Ready for FOB in 5-6 days from order placement.
- Price FOB China available on serious interest.
- Customs cleared.
- Country of origin – Global Sourcing.
- Logistics Options-
- Direct Charter
- Part Shipment Charter
- Combo of sea + air.
- FDA/CE
- EN ISO 15223-1-2016
- ISO-13485 : 2016
- EN-13612 : 2002
- EN ISO 17511 : 2001
- EN 23640 : 2015
- EN 13641 : 2002
- EN ISO 14971 : 2012
- EN ISO 18113 – 1 : 2011
- EN ISO 18113 – 2 : 2011
- IEC 62366 – 1 : 2015
- IEC 62366 – 2 : 2015
- The product is stable until the expiration dates stated on the box and aluminum foil bag labels, when sealed and stored at 4°C ~30°C and protected from direct sunlight.
- Avoid freezing.
- Each laboratory should determine the suitability of the blood collection tubes and serum isolation products it uses.
- Transport and store the samples under low temperature conditions.
- The samples can be stored for 7 days under the conditions of 2°C – 8°C.
- Serum or plasma samples can be stored for 3 months at -20°C, and repeated freeze-thaw cycles should not exceed 3.
- At ambient temperature (10°C~30°C), equilibrate the samples stored at low temperature for at least 30 minutes before use.







